Since the Nuremberg Code was established in 1947, patient safety has been a cornerstone of clinical trial design. The core principles of the Code mandate that trials must prioritize the well-being of participants, ensure informed consent, and allow patients the freedom to withdraw from studies at any time. Modern guidelines like ICH-GCP enforce these principles by obligating sponsors, contract research organizations (CROs), and investigators to maintain patient safety throughout the process. Incorporating patient perspectives into study design and execution is proving crucial for improving clinical trial outcomes, including recruitment and retention rates, which ultimately contribute to more effective treatments.
Historically, clinical trials have been driven primarily by scientific and regulatory demands, often at the expense of understanding patient needs. However, the industry is gradually shifting towards a more patient-centric approach, where patients are treated as key partners in the development of new therapies, rather than just participants in research studies.
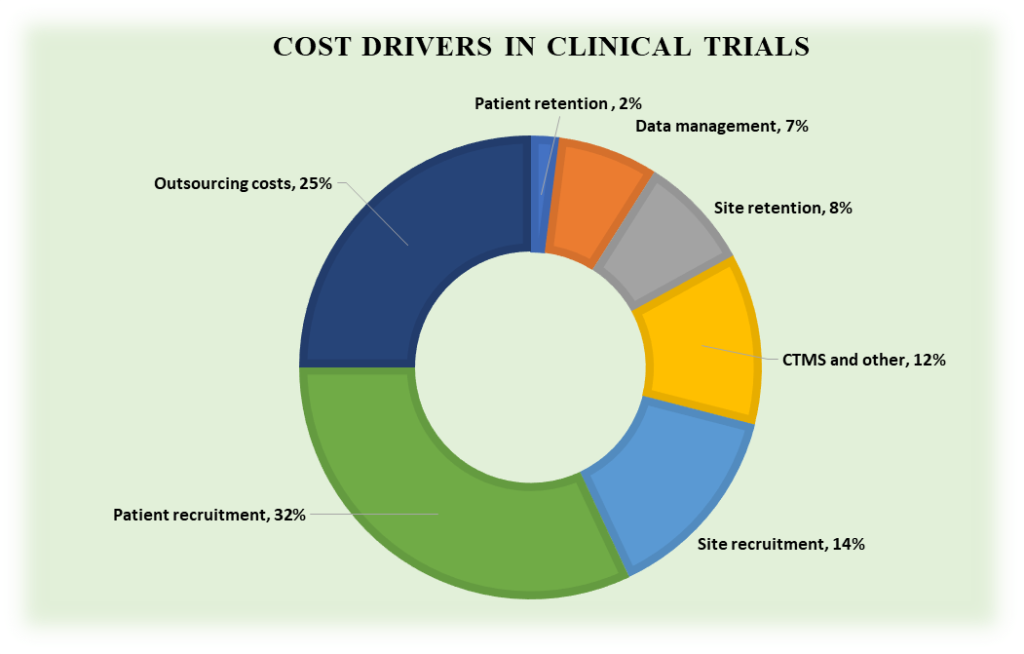
The traditional focus on meeting regulatory standards and scientific objectives has begun to evolve, recognizing that patient engagement is vital for creating more meaningful and impactful research outcomes. As regulators and industry stakeholders increasingly endorse this shift, patient-centric trials are becoming a vital part of the research landscape, improving the relevance of clinical studies and enhancing therapeutic development. This article delves into the concept of patient-centric clinical trials, examining their definition, advantages, methodologies, and future trajectory, and exploring how this approach is transforming research for better patient outcomes.
.
The Role of Patient Engagement
To create truly patient-centric trials, the research community is shifting its focus to understanding patients as individuals—recognizing their experiences, concerns, and preferences. Trials designed with a focus on outcomes that matter most to patients are key to making research more inclusive. This focus allows for a better alignment between the study’s goals and the real-life impact on patients’ daily lives.
1. Understanding Participant Needs and Barriers
One of the main challenges in clinical trials is recognizing and addressing the various barriers that prevent patients from enrolling or remaining in studies. These barriers include logistical issues such as time constraints, transportation difficulties, and financial burdens. By understanding these barriers, researchers can implement strategies that make participation more feasible.
1.1 Identifying Barriers
Recruitment and retention are often hindered by:
- Time constraints: Many patients have busy schedules due to work, family obligations, or other commitments.
- Transportation issues: Lack of reliable transportation to research sites can prevent participation, particularly for elderly individuals or those in rural areas.
- Financial concerns: Costs associated with travel, childcare, and lost wages are major deterrents.
1.2 Solutions for Overcoming Barriers
To overcome the barriers hindering participation in clinical trials, researchers can adopt practical, patient-centered solutions that address logistical, financial, and accessibility challenges.
· Flexible Appointment Scheduling
Many patients face time constraints due to work or personal commitments. Offering flexible appointment options, such as evening or weekend hours, can help accommodate their schedules. Additionally, quick appointment turnarounds and timely reminders can reduce the burden on participants.
· Travel and Childcare Reimbursements
Financial burdens, including travel, childcare, and lost wages, often discourage participation. Researchers can alleviate these concerns by providing reimbursements for transportation and childcare costs, as well as compensation for time off work. This makes participation more financially feasible for patients.
· Home Visits and Telemedicine
Home visits for follow-ups or data collection can significantly reduce the need for travel, especially for patients in rural areas or with mobility issues. Additionally, telemedicine allows participants to attend appointments remotely, making it easier for them to stay engaged in the trial without frequent visits to the clinic.
· Simplifying Enrollment and Administrative Processes
Streamlining the enrollment process by reducing paperwork and offering online registration options can make it easier for patients to join trials. Providing multilingual materials ensures that language is not a barrier for diverse populations.
· Providing Emotional and Social Support
Providing support through patient navigators, counseling, and support groups can help address emotional or psychological barriers. Personalized assistance and peer support can increase comfort and confidence, encouraging participation and retention.
By implementing these solutions, researchers can make clinical trials more accessible and inclusive, fostering greater patient engagement and improving recruitment and retention rates.
2. Building Trust and Enhancing Communication
Effective communication plays a central role in both recruitment and retention. Clear, transparent, and empathetic communication fosters trust between participants and researchers, encouraging enrolment and ongoing participation.
2.1 Trust as a Key Factor in Decision-Making
Patients who trust healthcare providers are more likely to engage in clinical trials. Trust can be built by:
- Clear and honest information: Providing participants with straightforward, easily understandable information about the study, including its purpose, potential risks, and benefits.
- Transparency in communication: Regular updates about the study’s progress and how the findings may impact their health or contribute to medical science.
2.2 Addressing Ambivalence
Many potential participants experience ambivalence—conflicting emotions about joining a clinical trial. Researchers can use strategies such as motivational interviewing (MI) to help patients navigate their feelings. MI techniques, such as open-ended questions and active listening, encourage patients to explore their concerns and make informed decisions without feeling pressured.
2.3 Effective Communication Framework
A conceptual framework for communication in clinical trials includes:
- Creating a safe, unpressured space: Ensuring that patients feel comfortable making decisions in their own time, without feeling rushed or judged.
- Building trust through transparency: Offering clear, honest answers to questions and addressing concerns directly.
- Providing opportunities for questions: Actively encouraging patients to voice their doubts or concerns, and being prepared to respond thoroughly.
3. Expanding Sociocultural Representation and Reach
A critical issue in clinical trials is the underrepresentation of minority groups. Historically, African American, Hispanic/Latinx, and other minority populations have been less likely to participate in clinical research. Addressing this underrepresentation is essential to ensure that clinical trials are inclusive and reflect the diversity of the patient population.
3.1 The Importance of Representation
Underrepresentation can lead to a lack of generalizability in trial results, ultimately impacting the effectiveness of treatments across diverse populations. Surveys and studies show that minority patients are more likely to trust clinical trials if they are introduced by individuals who share their cultural background.
3.2 Strategies for Increasing Diversity
- Culturally sensitive recruitment: Employing bilingual staff, using culturally relevant materials, and ensuring that recruitment strategies are aligned with the values of the target community.
- Engaging community-based resources: Partnering with local community leaders, health organizations, and trusted figures to facilitate recruitment. This might involve presenting information through community health fairs, churches, and local events.
- Community advisory panels: Establishing advisory panels made up of community members who can help design studies that meet the cultural needs of underrepresented groups.
3.3 Decentralization and Digital Tools
The COVID-19 pandemic accelerated the trend toward decentralization of clinical trials. Utilizing digital technologies can reduce geographic constraints and make trials more accessible to a broader population. Online recruitment, remote data collection, and telemedicine are tools that can help reach underrepresented groups more effectively. However, the digital divide remains a challenge, and efforts should be made to ensure that technology use does not exclude patients who lack access to digital resources.
4. Multi-Pronged Recruitment Strategies
A one-size-fits-all approach to recruitment is often ineffective. Instead, a multi-pronged strategy that combines traditional and modern methods can reach a diverse group of potential participants.
4.1 Building Relationships with Healthcare Providers
Collaboration with primary care physicians, community health providers, and specialists is essential to enhance recruitment efforts. Patients are more likely to trust information about clinical trials when it comes from a healthcare provider they already trust. By building strong relationships with healthcare professionals, researchers can leverage their networks for referrals.
4.2 Utilizing Multiple Platforms for Outreach
Research has shown that using a variety of outreach methods improves recruitment:
- Online and social media: Platforms like social media, websites, and email campaigns allow researchers to reach a large audience quickly and cost-effectively.
- Traditional methods: Flyers, posters in waiting rooms, and community events also play an important role, particularly for reaching those who may not be active online.
4.3 Incentivizing Participation
Offering incentives such as financial compensation or access to healthcare services may increase interest and motivate participation. Furthermore, incentives can help reduce the financial burden associated with clinical trial participation.
5. Retention Strategies: Ensuring Ongoing Engagement
While recruitment is essential, maintaining participant engagement throughout the study is just as important. Retention strategies help ensure that participants stay committed to the trial, even when faced with challenges or setbacks.
5.1 Enhancing Competence and Relatedness
- Providing information and education: Ensuring that participants understand their role in the study and the impact of their contribution helps foster a sense of purpose.
- Personalized communication: Maintaining regular contact with participants, including thank-you letters, progress updates, and reminders, helps build rapport and encourages continued engagement.
5.2 Addressing Participant Concerns
Researchers should remain vigilant for signs of disengagement, such as missed appointments or lack of communication. Offering flexibility, additional support, and addressing any concerns promptly can help prevent attrition.
5.3 Maintaining Organizational Competence
An environment that is clean, accessible, and supportive reflects the value researchers place on participant engagement. Staff should be well-trained in handling patient interactions with empathy, respect, and professionalism.
Conclusion
Recruitment and retention in clinical trials are crucial for the advancement of medical science. By adopting a patient-centric approach, researchers can improve engagement, foster trust, and address the diverse needs of participants. Implementing flexible scheduling, leveraging digital technologies, communicating effectively, and embracing cultural sensitivity are just a few of the strategies that can enhance recruitment and retention. Ultimately, making clinical trials more accessible and aligned with patient needs will not only improve the quality and diversity of trial outcomes but also contribute to the development of treatments that are effective for all populations.




